Partnering to Advance
Liver Cancer Care


In hepatocellular carcinoma (HCC), the most common type of liver cancer, a significant number of patients are diagnosed in the early stage, providing the potential for a cure through ablation or resection. However, the lack of effective drugs to prevent HCC recurrence after resection underscores the critical need for potential adjuvant therapy options for this population.
- Liver Cancer
- 6thmost common cancer [1]
- 1million +
- global cases annually
- About 30% of patient eligible for resection or transplantation[2]
- 70% recurrence rates
- in 5 year [3]
- Clinical Unmet Needs High
- : no approved therapy for
- adjuvant setting[4]
- [1]Llovet, J. M., Kelley, Hepatocellular carcinoma. Nature reviews. Disease primers, 7(1), 6.
- [2]Hepatocellular carcinoma. Nature reviews. Disease primers, 7(1), 6.
- [3]N Engl J Med. 2019 Apr 11;380(15):1450-1462
- [4]NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatocellular Carcinoma, Version 1.2023



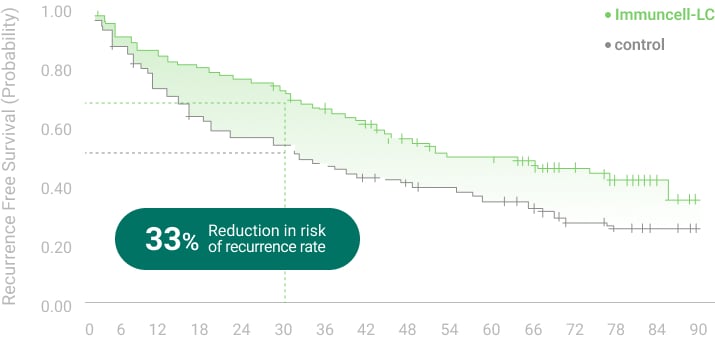
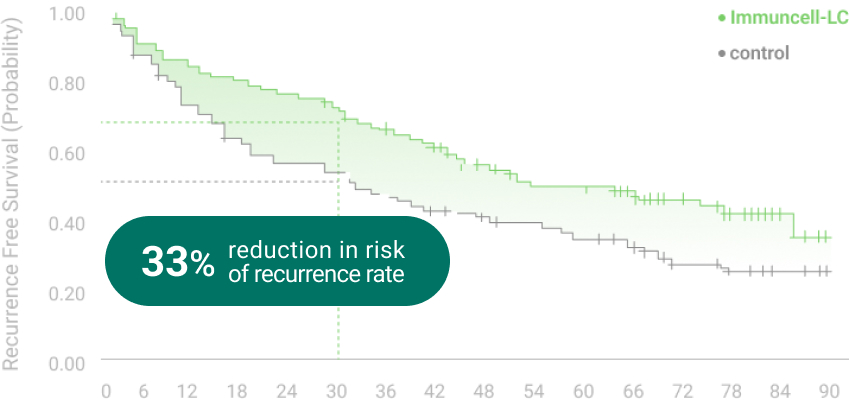
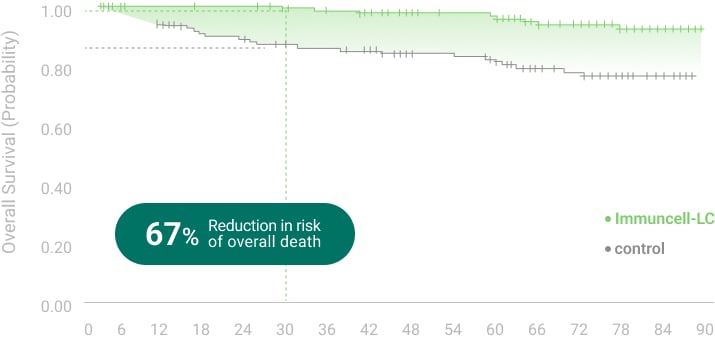
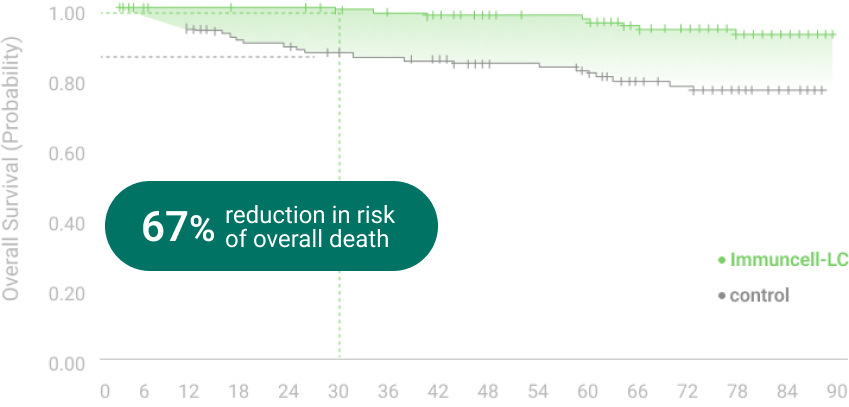
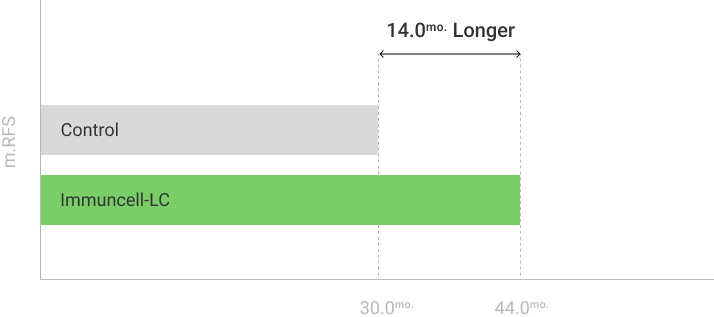
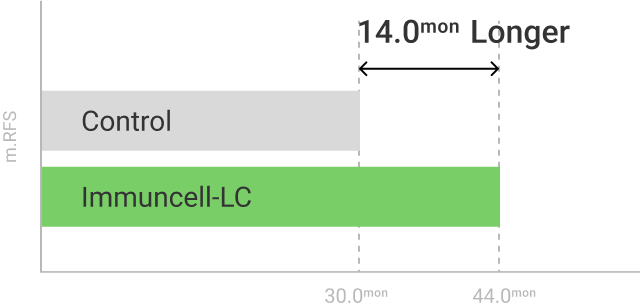
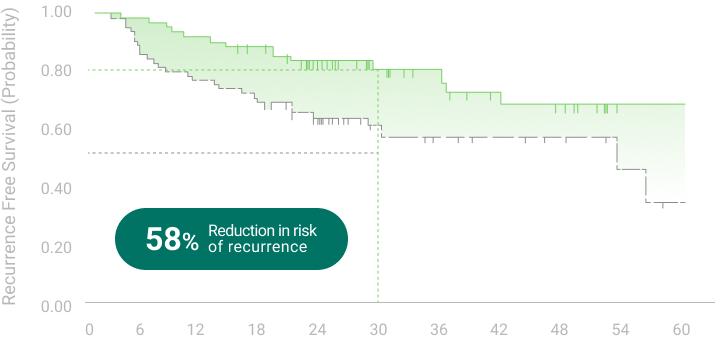
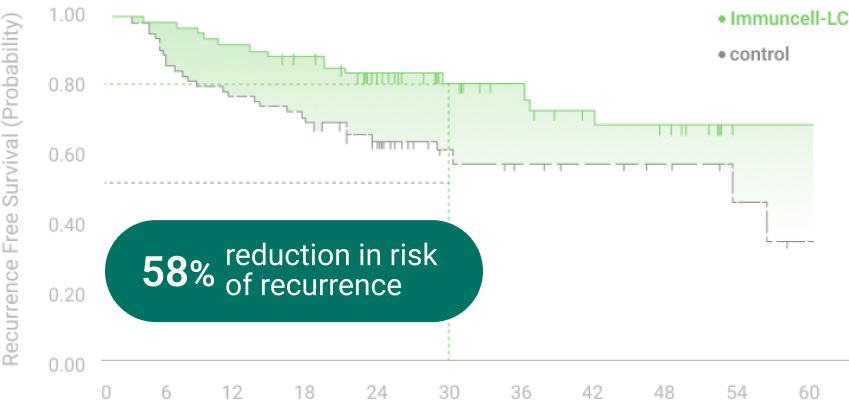
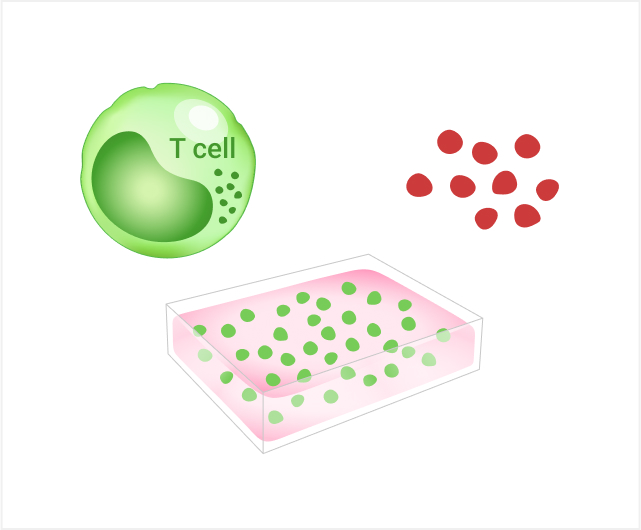
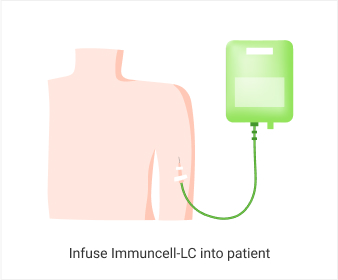
The 5 years follow-up starts with the enrollment of last patient.
** RFS: Recurrence Free Survival *** OS: Overall Survival
Ref.1. Gastroenterology (2015) 148:1383-1391,
2. Cancer Immunol Immunother. (2019) 68(1): 23-32,
3. NCT00699816